A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
...

Chemistry, 26.10.2021 19:30 lazavionadams81
A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
AH tus water = 334 J/g
Cu = 0.89 J/gºC
What is the final temperature of both substances?
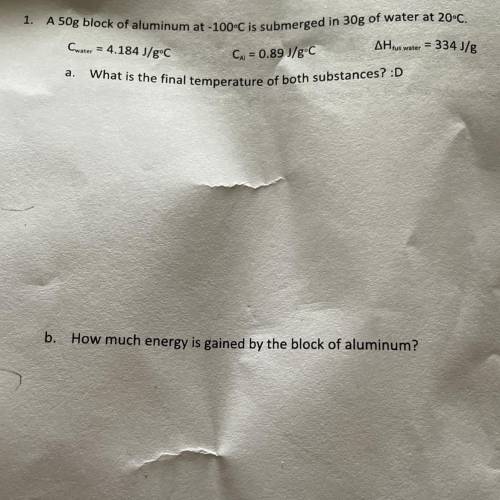

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Questions

Mathematics, 10.08.2021 14:00

Computers and Technology, 10.08.2021 14:00

English, 10.08.2021 14:00


English, 10.08.2021 14:00

Physics, 10.08.2021 14:00

Mathematics, 10.08.2021 14:00

Mathematics, 10.08.2021 14:00



Mathematics, 10.08.2021 14:00



Mathematics, 10.08.2021 14:00



Biology, 10.08.2021 14:00

Social Studies, 10.08.2021 14:00


Advanced Placement (AP), 10.08.2021 14:00



