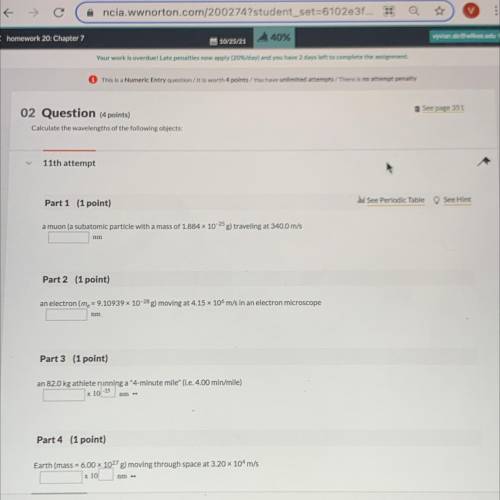
Calculate the wavelengths of the following objects:
Part 1 (1 point)
See Periodic Table See...

Chemistry, 25.10.2021 23:20 babyboogrocks5695
Calculate the wavelengths of the following objects:
Part 1 (1 point)
See Periodic Table See Hint
a muon (a subatomic particle with a mass of 1.884 x 10-25 g) traveling at 340.0 m/s
nm
Part 2 (1 point)
an electron (me = 9.10939 x 10-28 g) moving at 4.15 x 106 m/s in an electron microscope
nm
Part 3 (1 point)
an 82.0 kg athlete running a "4-minute mile" (i. e. 4.00 min/mile)
x 10-25
nm
Part 4 (1 point)
Earth (mass = 6.00 x 10278) moving through space at 3.20 x 10m/s
x 10
nm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 11:30
All of the following describe uses of nonrenewable energy sources except
Answers: 3
You know the right answer?
Questions

Health, 22.10.2019 00:00


Mathematics, 22.10.2019 00:00


Chemistry, 22.10.2019 00:00

History, 22.10.2019 00:00



Spanish, 22.10.2019 00:00

Social Studies, 22.10.2019 00:00


History, 22.10.2019 00:00

History, 22.10.2019 00:00

Spanish, 22.10.2019 00:00

History, 22.10.2019 00:00



History, 22.10.2019 00:00

Mathematics, 22.10.2019 00:00



