
Chemistry, 25.10.2021 18:00 angelinaavila06
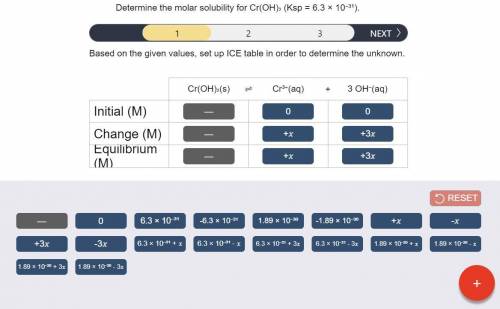
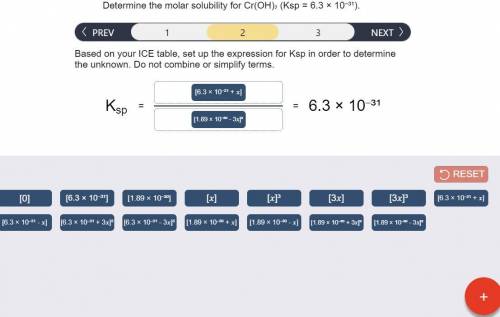
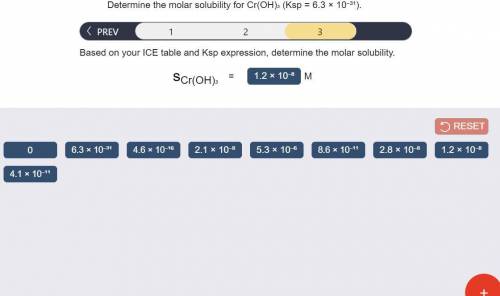
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s) <-> Cr^3+ (aq) + 3OH^- (aq)
b) Ksp expression
c) Determine molar solubility

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s)...
Questions

History, 08.10.2019 07:30

Biology, 08.10.2019 07:30

Physics, 08.10.2019 07:30

Biology, 08.10.2019 07:30


Social Studies, 08.10.2019 07:30



Mathematics, 08.10.2019 07:30

English, 08.10.2019 07:30


Social Studies, 08.10.2019 07:30

History, 08.10.2019 07:30

English, 08.10.2019 07:30



History, 08.10.2019 07:30


Physics, 08.10.2019 07:30

Arts, 08.10.2019 07:30



