
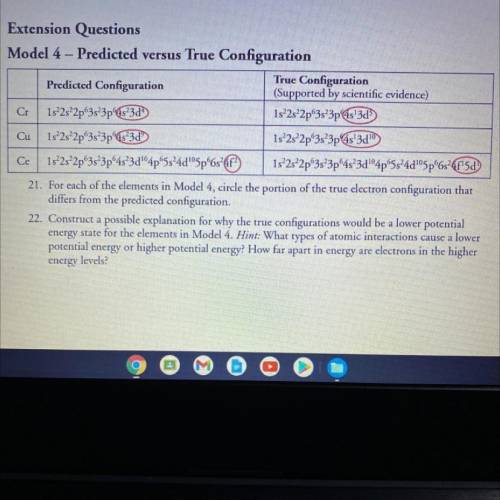
Construct a possible explanation for why true configurations would be a lower potential energy state for the elements in Model 4. Hint: What types of atomic interactions cause a lower potential energy or higher potential energy? How far apart in energy are electrons in the higher energy levels? Please help me with this question, thank you

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1


Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Construct a possible explanation for why true configurations would be a lower potential energy state...
Questions


Mathematics, 14.12.2020 19:20


Mathematics, 14.12.2020 19:20

Mathematics, 14.12.2020 19:20


Mathematics, 14.12.2020 19:20



Mathematics, 14.12.2020 19:20








Mathematics, 14.12.2020 19:20

History, 14.12.2020 19:20




