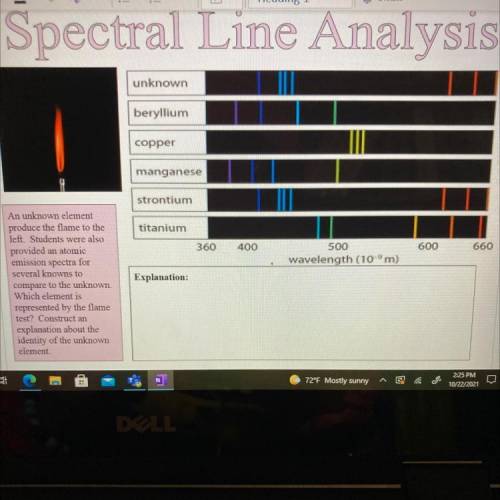
An unknown element
produce the flame to the
left. Students were also
provided an atomi...

Chemistry, 24.10.2021 08:20 jennelledenise
An unknown element
produce the flame to the
left. Students were also
provided an atomic
emission spectra for
several knowns to
compare to the unknown.
Which element is
represented by the flame
test? Construct an
explanation about the
identity of the unknown
element.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Questions


Biology, 20.08.2021 20:40

English, 20.08.2021 20:40


Biology, 20.08.2021 20:40


English, 20.08.2021 20:40



Arts, 20.08.2021 20:40

Mathematics, 20.08.2021 20:40


Mathematics, 20.08.2021 20:40



Mathematics, 20.08.2021 20:40

Mathematics, 20.08.2021 20:40

Mathematics, 20.08.2021 20:40





