
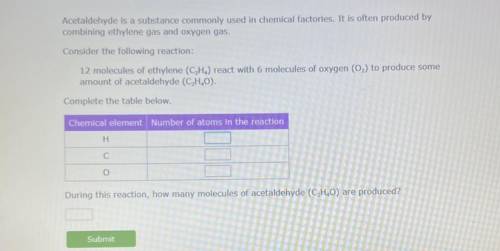
12 molecules of ethylene
(C2H4) react with 6 molecules of oxygen (O2) to produce some amount of acetaldehyde. (C2H4O)
During this reaction how many molecules of acetaldehyde (C2H4O) are produced?
How many atoms are in each reactant also? H, C, and O.
View photo for more info.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
12 molecules of ethylene
(C2H4) react with 6 molecules of oxygen (O2) to produce some amount of ac...
Questions



Mathematics, 23.11.2020 08:50

Social Studies, 23.11.2020 08:50

Mathematics, 23.11.2020 08:50

English, 23.11.2020 08:50

History, 23.11.2020 08:50




Mathematics, 23.11.2020 08:50

Mathematics, 23.11.2020 08:50

English, 23.11.2020 08:50

Physics, 23.11.2020 08:50



Mathematics, 23.11.2020 08:50



Mathematics, 23.11.2020 08:50



