
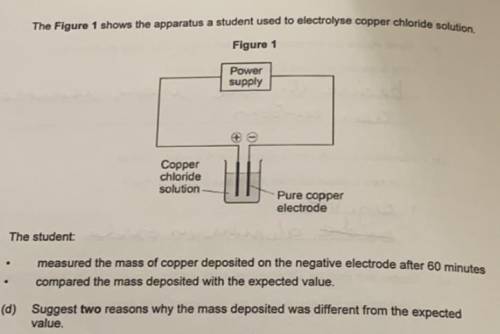
The Figure 1 shows the apparatus a student used to electrolyse copper chloride solution
The student:
- measured the mass of copper deposited on the negative electrode after 60 minutes
- compared the mass deposited with the expected value.
Suggest two reasons why the mass deposited was different from the expected
value.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
The Figure 1 shows the apparatus a student used to electrolyse copper chloride solution
The studen...
Questions

Social Studies, 05.11.2020 23:20

Biology, 05.11.2020 23:20



Chemistry, 05.11.2020 23:20



History, 05.11.2020 23:20



Health, 05.11.2020 23:20

English, 05.11.2020 23:20

Mathematics, 05.11.2020 23:20



History, 05.11.2020 23:20



Mathematics, 05.11.2020 23:20



