
Chemistry, 18.10.2021 21:40 lovvyDovvy04
According to the law of conservation of matter, we know that the total number of atoms does not change in a chemical reaction and thus mass is conserved. This is part of a chemical reaction: hydrogen plus oxygen yields water. Can you complete this model? Reorganize the reactants in order to complete the product side of the reaction.
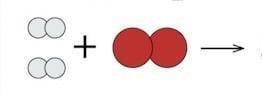





Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
According to the law of conservation of matter, we know that the total number of atoms does not chan...
Questions

Biology, 21.10.2019 22:20


Mathematics, 21.10.2019 22:20


Biology, 21.10.2019 22:20

Mathematics, 21.10.2019 22:20




Mathematics, 21.10.2019 22:20

Mathematics, 21.10.2019 22:20


Mathematics, 21.10.2019 22:20

Health, 21.10.2019 22:20





Mathematics, 21.10.2019 22:20

Mathematics, 21.10.2019 22:20



