
Chemistry, 18.10.2021 05:10 zamariahyou
Hii I need help ASAP - 100 points!!!
Energy and Enthalpy Changes, Heat and Work -- Monatomic Ideal Gas
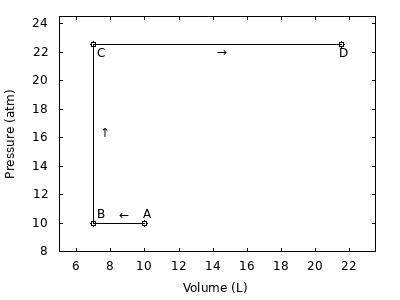
*See picture
2.00-mol of a monatomic ideal gas goes from State A to State D via the path A→B→C→D:
State A PA=10.0atm, VA=10.00L
State B PB=10.0atm, VB=7.00L
State C PC=22.5atm, VC=7.00L
State D PD=22.5atm, VD=21.50L
1. Assume that the external pressure is constant during each step and equals the final pressure of the gas for that step.
Calculate q for this process
2. Calculate w for this process.
3. Calculate ΔE for this process
4. Calculate ΔH for this process.
*If you can explain the steps great! I would really appreciate it, if not just the answers are also fine

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Hii I need help ASAP - 100 points!!!
Energy and Enthalpy Changes, Heat and Work -- Monatomic Ideal...
Questions

Mathematics, 29.08.2019 18:30


History, 29.08.2019 18:30

Mathematics, 29.08.2019 18:30

Mathematics, 29.08.2019 18:30


History, 29.08.2019 18:30

History, 29.08.2019 18:30








Mathematics, 29.08.2019 18:30

Mathematics, 29.08.2019 18:30





