
Chemistry, 17.10.2021 14:00 Nayerlin16
Calculate the molality of 44.6% by mass aqeous solution of barrium chloride the molar mass of barrium chloride is 208g/mol
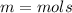

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Calculate the molality of 44.6% by mass aqeous solution of barrium chloride the molar mass of barriu...
Questions


Mathematics, 21.05.2021 16:30

Computers and Technology, 21.05.2021 16:30

Mathematics, 21.05.2021 16:30


Chemistry, 21.05.2021 16:30

Mathematics, 21.05.2021 16:30

Mathematics, 21.05.2021 16:30

Mathematics, 21.05.2021 16:30


Mathematics, 21.05.2021 16:30

English, 21.05.2021 16:30

Law, 21.05.2021 16:30




Mathematics, 21.05.2021 16:30

Mathematics, 21.05.2021 16:30




