
Chemistry, 14.10.2021 01:00 abelinoperez652
if 1.40 mol mg are mixed with 2.00 mol of HCl, how many moles of excess reactant remain after the reaction is complete?
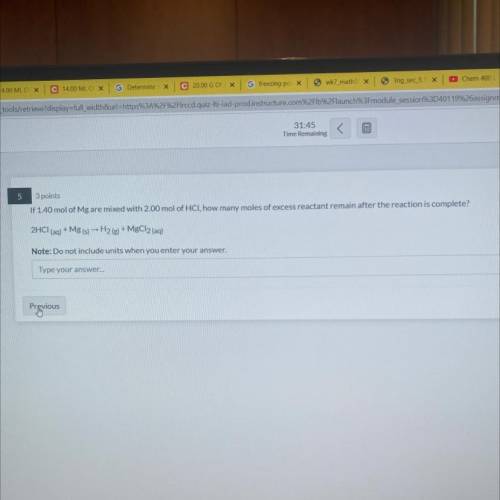

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2


Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
You know the right answer?
if 1.40 mol mg are mixed with 2.00 mol of HCl, how many moles of excess reactant remain after the re...
Questions



Advanced Placement (AP), 24.09.2019 09:50

History, 24.09.2019 09:50




Business, 24.09.2019 09:50

Geography, 24.09.2019 09:50

Chemistry, 24.09.2019 09:50

Social Studies, 24.09.2019 09:50

Health, 24.09.2019 09:50

Mathematics, 24.09.2019 09:50


Mathematics, 24.09.2019 09:50

English, 24.09.2019 09:50

SAT, 24.09.2019 09:50

Health, 24.09.2019 09:50




