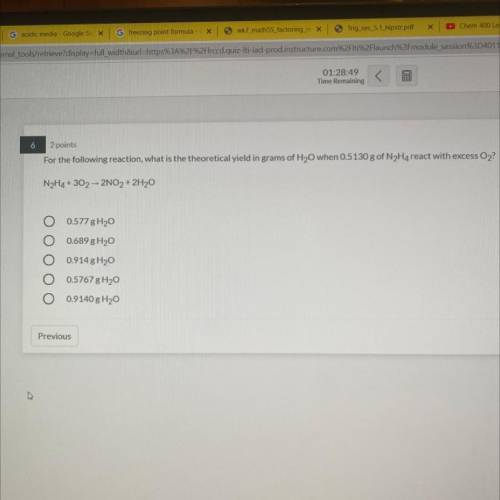
6
2 points
For the following reaction, what is the theoretical yield in grams of H2O when 05...

Chemistry, 13.10.2021 14:00 noberoger2780
6
2 points
For the following reaction, what is the theoretical yield in grams of H2O when 05130 g of N Ha react with excess O2
NH4+ 30, 2NO, + 2H30
O 0577 8H20
0.689 H2O
ООООО
0914 8H2O
O 0.5767 g 120
O 0.9140 g Hy

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
Questions



Computers and Technology, 26.02.2020 19:50













Biology, 26.02.2020 19:51



Mathematics, 26.02.2020 19:52

Mathematics, 26.02.2020 19:52



