
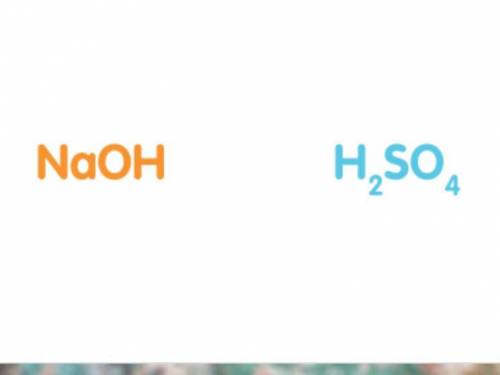
50cm3 of sodium hydroxide solution was titrated against a solution of sulfuric acid. The concentration of the sodium hydroxide solution was 20g/dm3. Work out the concentration of the acid in moles per litre if it took 25cm3 of acid to completely neutralise the alkali. (Hint: It may help to write out a balanced symbol equation for the reaction. The chemical formulas of the acid and alkali are shown below.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
50cm3 of sodium hydroxide solution was titrated against a solution of sulfuric acid. The concentrati...
Questions

English, 25.09.2019 10:30


Mathematics, 25.09.2019 10:30

Mathematics, 25.09.2019 10:30


Computers and Technology, 25.09.2019 10:30



English, 25.09.2019 10:30



Mathematics, 25.09.2019 10:30

History, 25.09.2019 10:30

Biology, 25.09.2019 10:30


Mathematics, 25.09.2019 10:30



Physics, 25.09.2019 10:30

Social Studies, 25.09.2019 10:30



