
ATOMS-
1. an atom is the smallest part of a pure substance which retains the _ of that pure substance
COMPOUNDS-
2. atoms of each element are essentially the _
LAW OF DEFINITE PROPORTIONS-
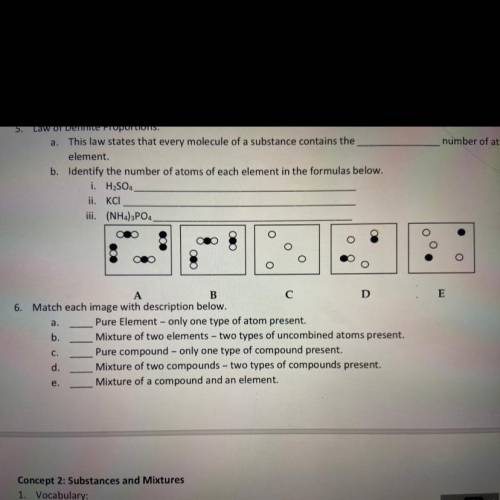
3. this law states that every molecule of a substance contains the _ number of atoms of each element
SOLUTIONS-
4. Solutions are special types of mixtures which are _ so they often can be confused as pure substances
STATES OF MATTER-
5. how would you describe the change in arrangement of particles as heat energy and temperature increases?
6. why does the temperature remain constant during a phase change even though the substance is absorbing heat energy?
7. (number six only)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
ATOMS-
1. an atom is the smallest part of a pure substance which retains the _ of that pure substa...
Questions


Social Studies, 06.12.2021 14:00

Mathematics, 06.12.2021 14:00

English, 06.12.2021 14:00





Mathematics, 06.12.2021 14:00


History, 06.12.2021 14:00








Biology, 06.12.2021 14:00

Physics, 06.12.2021 14:00



