
Chemistry, 10.10.2021 03:20 olejlund8073
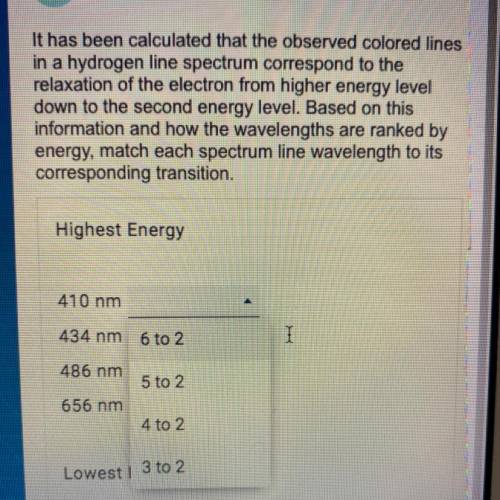
It has been calculated that the observed colored lines in a hydrogen line spectrum correspond to the relaxation of the electron from higher energy level down to the second energy level. Based on this information and how the wavelengths are ranked by energy, match each spectrum line wavelength to its corresponding transition.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

You know the right answer?
It has been calculated that the observed colored lines in a hydrogen line spectrum correspond to the...
Questions

Social Studies, 19.07.2019 18:50





Physics, 19.07.2019 18:50

Arts, 19.07.2019 18:50

Health, 19.07.2019 18:50

Arts, 19.07.2019 18:50

History, 19.07.2019 18:50


Business, 19.07.2019 18:50

Biology, 19.07.2019 18:50

Biology, 19.07.2019 18:50

Biology, 19.07.2019 18:50

History, 19.07.2019 18:50

Business, 19.07.2019 18:50

Mathematics, 19.07.2019 18:50

Business, 19.07.2019 18:50

Biology, 19.07.2019 18:50



