
Chemistry, 09.10.2021 14:00 magmoo3779
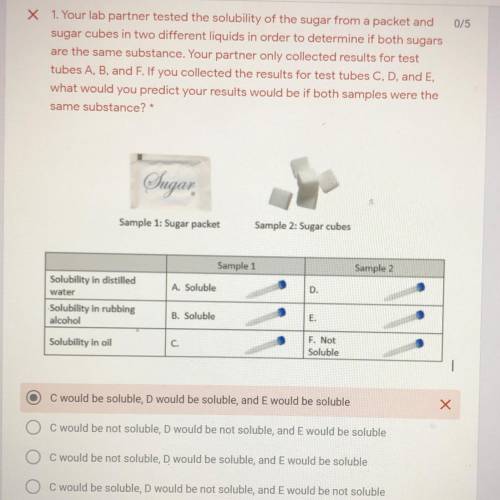
Your lab partner tested the solubility of a sugar from a packet and sugar cubes in two different liquids in order to determine if both sugars are the same substance. Your partner only collected the results for the test tubes A, B, and F. If you collected the results for C D and E, what would you predict your results would be if both samples were the same substance
A. C would be soluble, D would be soluble, and E would be soluble
B. C would be not soluble, D would be not soluble, and E would be soluble
C. C would be not soluble, D would be soluble, and E would be soluble.
D. C would be soluble, D would not be soluble, and E would be not soluble

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
Your lab partner tested the solubility of a sugar from a packet and sugar cubes in two different liq...
Questions



English, 30.08.2019 14:10

Mathematics, 30.08.2019 14:10


Biology, 30.08.2019 14:10

Mathematics, 30.08.2019 14:10




Social Studies, 30.08.2019 14:10

Biology, 30.08.2019 14:10


History, 30.08.2019 14:10

Biology, 30.08.2019 14:10


English, 30.08.2019 14:10

Biology, 30.08.2019 14:10




