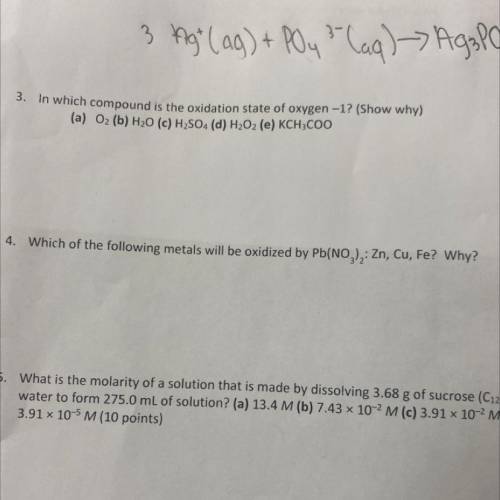Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
3. In which compound is the oxidation state of oxygen -1? (Show why)
(a) 02 (b) H20 (c) H2SO4 (d)...
Questions

Mathematics, 13.01.2020 09:31


Mathematics, 13.01.2020 09:31

English, 13.01.2020 09:31

Physics, 13.01.2020 09:31


Health, 13.01.2020 09:31

Computers and Technology, 13.01.2020 09:31

English, 13.01.2020 09:31



Mathematics, 13.01.2020 09:31

Social Studies, 13.01.2020 09:31

History, 13.01.2020 09:31


Physics, 13.01.2020 09:31