
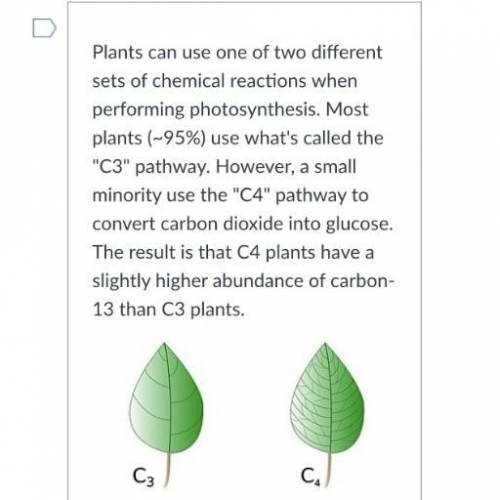
Use the image attached to answer the following questions ASAP please!!
⍣Honey (made from C3 plant sources) has the following C - 12 / C - 13 abundances. Calculate the average atomic mass of carbon in honey -12:98.934896 -131.0652 %
⍣ How much would 42, 000, 0m moles of glucose (C_{6}*H_{12} * O_{6}) extracted from honey weigh in grams? (Assume oxygen and hydrogen have their normal atomic mass.)
⍣ A 1.238756 mole sample of sugar from a jelly sample weighs 223,1716910 g this jelly most likely sweetened with sugar sugar cane or with honey ? Explain your reasoning.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2
You know the right answer?
Use the image attached to answer the following questions ASAP please!!
⍣Honey (made from C3 plant...
Questions



English, 01.01.2020 01:31

Mathematics, 01.01.2020 01:31

English, 01.01.2020 01:31


Mathematics, 01.01.2020 01:31


Mathematics, 01.01.2020 01:31


Mathematics, 01.01.2020 01:31



Mathematics, 01.01.2020 01:31





History, 01.01.2020 01:31

History, 01.01.2020 01:31



