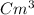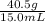Chemistry, 04.10.2021 20:30 Victoriag2626
50 Points Help
1) A block of aluminum occupies a volume of 15.0 mL and mass 40.5 g. What is its density?
2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder mass 306.0 g. From this information, calculate the density of mercury.
3) What is the mass of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL.
4) A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, what is the density of copper? ( hint find the volume first: volume of rectangle = Hight x width x length)
5) Calculate the density of sulfuric acid if 35.4 mL of the acid mass 65.14 g.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
50 Points Help
1) A block of aluminum occupies a volume of 15.0 mL and mass 40.5 g. What is its de...
Questions





English, 17.09.2021 14:00

English, 17.09.2021 14:00


Geography, 17.09.2021 14:00








Mathematics, 17.09.2021 14:00


Mathematics, 17.09.2021 14:00


Biology, 17.09.2021 14:00

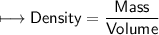

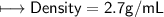

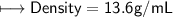

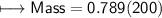
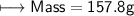
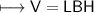
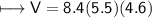
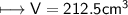
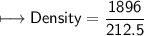
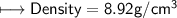
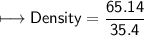
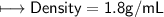
 when dealing with a solid.
when dealing with a solid.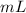 or
or