Chemistry, 04.10.2021 15:00 bestsonever698
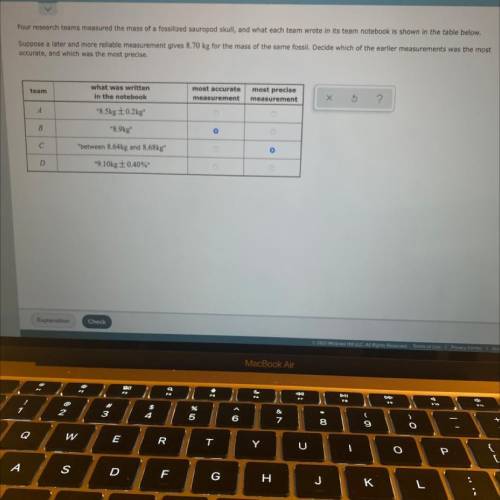
Four research teams measured the mass of fossilized sauropod skull, and what each team wrote in its team notebook is shown in the table below,
Suppose a later and more reliable measurement gives 8.70 kg for the mass of the same fossil, Dedde which of the earlier measurements was the most
accurate, and which was the most precise
team
what was written
in the notebook
*8,5kg 0.2kg
most accurate
measurement
most precise
measurement
G
x
2
}
*8.9ky
C
between 8/Ake and 8.68kg
D
9.10kg 0 40%
Exploration
Check

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

You know the right answer?
Four research teams measured the mass of fossilized sauropod skull, and what each team wrote in its...
Questions

Mathematics, 24.11.2020 20:30

History, 24.11.2020 20:30

Biology, 24.11.2020 20:30





Mathematics, 24.11.2020 20:30



Mathematics, 24.11.2020 20:30

Mathematics, 24.11.2020 20:30


English, 24.11.2020 20:30

Mathematics, 24.11.2020 20:30



History, 24.11.2020 20:30