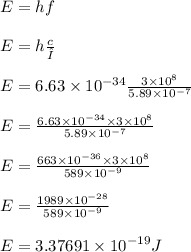Calculate the energy of one photon of yellow light that has a wavelength of 5.89x10^-7m.
...

Chemistry, 04.10.2021 08:40 Lilabsterdoll
Calculate the energy of one photon of yellow light that has a wavelength of 5.89x10^-7m.


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
Questions


History, 30.10.2019 14:31

Mathematics, 30.10.2019 14:31

Mathematics, 30.10.2019 14:31


Mathematics, 30.10.2019 14:31

Health, 30.10.2019 14:31

Mathematics, 30.10.2019 14:31



Biology, 30.10.2019 14:31






Mathematics, 30.10.2019 14:31

Mathematics, 30.10.2019 14:31

English, 30.10.2019 14:31

Chemistry, 30.10.2019 14:31