
Chemistry, 03.10.2021 01:00 memoryofdale
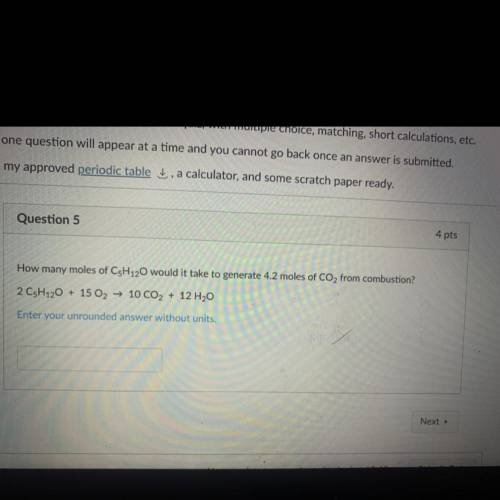
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15 O2 → 10 CO2 + 12 H2O
Enter your unrounded answer without units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15...
Questions


Mathematics, 05.05.2021 17:30

Mathematics, 05.05.2021 17:30



Social Studies, 05.05.2021 17:30



History, 05.05.2021 17:30



Chemistry, 05.05.2021 17:30


Mathematics, 05.05.2021 17:30

English, 05.05.2021 17:30


Mathematics, 05.05.2021 17:30

Mathematics, 05.05.2021 17:30


Mathematics, 05.05.2021 17:30



