carbon

Chemistry, 01.10.2021 06:30 Serenitybella
Look at the models of molecules below. Select all the compounds.
carbon monoxide
carbon
tetrachloride
phosphorus
tribromide
bromomethane
chloromethanol
water
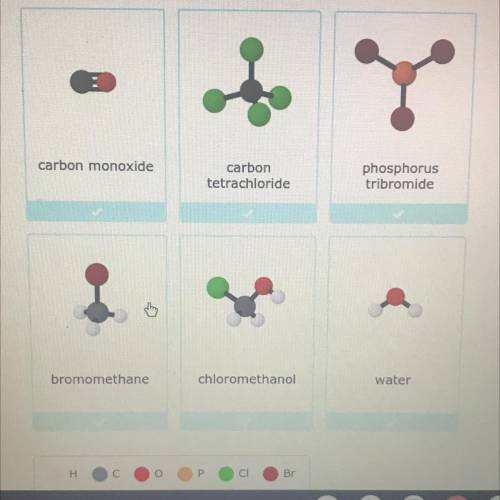

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Look at the models of molecules below. Select all the compounds.
carbon monoxide
carbon
carbon
Questions

Mathematics, 31.03.2021 17:50

Mathematics, 31.03.2021 17:50

Mathematics, 31.03.2021 17:50


English, 31.03.2021 17:50


Mathematics, 31.03.2021 17:50


Mathematics, 31.03.2021 17:50


Social Studies, 31.03.2021 17:50

Computers and Technology, 31.03.2021 17:50


Biology, 31.03.2021 17:50

English, 31.03.2021 17:50


Mathematics, 31.03.2021 17:50






