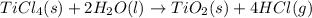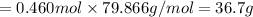Chemistry, 29.09.2021 21:10 kobiemajak
When TiCl4 (s) reacts with H20 (l), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g of
titanium (IV) chloride present, with water in excess, how much solid titanium (IV) oxide (in grams)
could theoretically be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
When TiCl4 (s) reacts with H20 (l), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g...
Questions

History, 30.01.2020 17:47

Physics, 30.01.2020 17:47

Mathematics, 30.01.2020 17:47




Mathematics, 30.01.2020 17:47


History, 30.01.2020 17:47

Chemistry, 30.01.2020 17:47


Mathematics, 30.01.2020 17:47


Mathematics, 30.01.2020 17:47

Biology, 30.01.2020 17:47

Mathematics, 30.01.2020 17:47

Mathematics, 30.01.2020 17:47


Mathematics, 30.01.2020 17:47