1 point
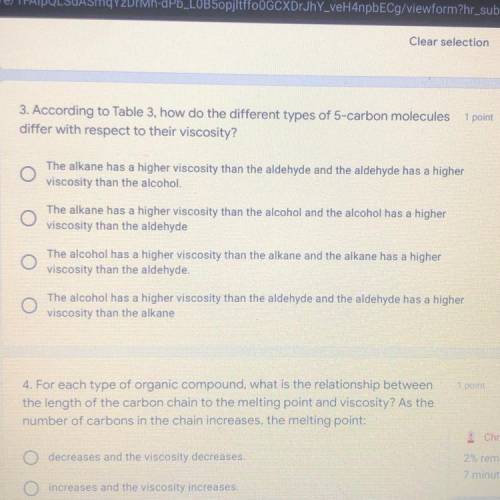
3. According to Table 3, how do the different types of 5-carbon molecules
differ wit...

Chemistry, 29.09.2021 19:20 nonjabulomabaso6850
1 point
3. According to Table 3, how do the different types of 5-carbon molecules
differ with respect to their viscosity?
The alkane has a higher viscosity than the aldehyde and the aldehyde has a higher
viscosity than the alcohol.
The alkane has a higher viscosity than the alcohol and the alcohol has a higher
viscosity than the aldehyde
The alcohol has a higher viscosity than the alkane and the alkane has a higher
viscosity than the aldehyde.
The alcohol has a higher viscosity than the aldehyde and the aldehyde has a higher
viscosity than the alkane

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

You know the right answer?
Questions

History, 28.01.2021 14:00

Mathematics, 28.01.2021 14:00



Mathematics, 28.01.2021 14:00

Mathematics, 28.01.2021 14:00

Mathematics, 28.01.2021 14:00


Mathematics, 28.01.2021 14:00



History, 28.01.2021 14:00


Advanced Placement (AP), 28.01.2021 14:00

Mathematics, 28.01.2021 14:00



Mathematics, 28.01.2021 14:00




