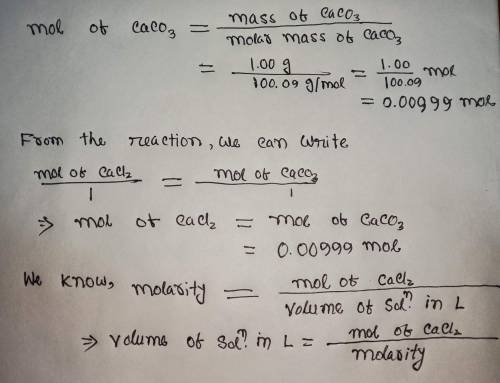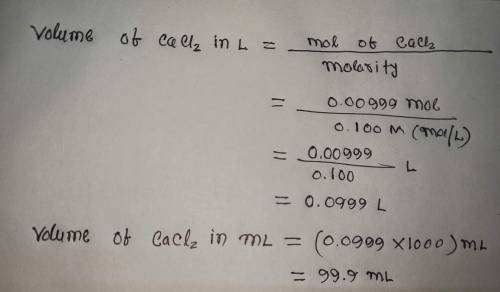Calculate the volume (in mL) of 0.100 M Na, CO3 needed to produce 1.00 g of
CaCO3(s)
. There...

Chemistry, 28.09.2021 03:10 hammackkatelyn60
Calculate the volume (in mL) of 0.100 M Na, CO3 needed to produce 1.00 g of
CaCO3(s)
. There is an excess of CaCl2.
Molar mass of calcium carbonate = 100.09 g/mol
*The answer is not 100ml or 10ml. Somehow the rounding up is not working well.*


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Questions





Spanish, 22.03.2021 06:20

Social Studies, 22.03.2021 06:20

Mathematics, 22.03.2021 06:20


Mathematics, 22.03.2021 06:20

Biology, 22.03.2021 06:20

Mathematics, 22.03.2021 06:20

Social Studies, 22.03.2021 06:20

Mathematics, 22.03.2021 06:20

History, 22.03.2021 06:20

Business, 22.03.2021 06:20

Mathematics, 22.03.2021 06:20


Mathematics, 22.03.2021 06:20


Mathematics, 22.03.2021 06:20