
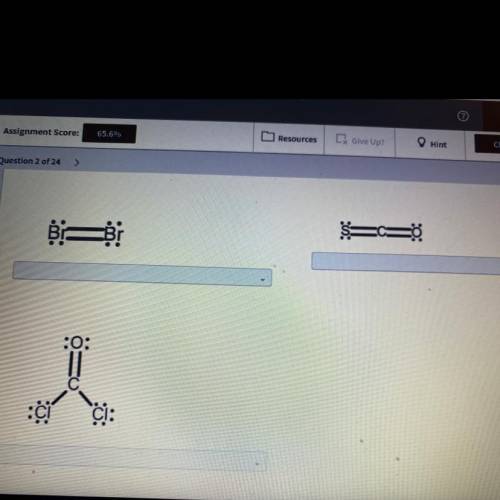
The ocular rule states that Adams in molecules share electrons in such a way that each atom has a full valence shell determine whether each structure has the correct number of electrons and obeys the ocular rule classify the structures that have the correct number of electrons and obey the ocular rule as valid and those that do not as invalid

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1


Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
The ocular rule states that Adams in molecules share electrons in such a way that each atom has a fu...
Questions





Social Studies, 19.10.2019 02:30

Social Studies, 19.10.2019 02:30


History, 19.10.2019 02:30


Chemistry, 19.10.2019 02:30

English, 19.10.2019 02:30




Computers and Technology, 19.10.2019 02:30

Mathematics, 19.10.2019 02:30




Advanced Placement (AP), 19.10.2019 02:30



