
Chemistry, 27.09.2021 14:00 xscape0mari
The molar mass of benzoic acid (C6H5COOH) determined by measuring the freezing‐point depression in benzene is twice what we would expect for the molecular formula, C7H6O2. Explain this apparent anomaly. Thank you! :)
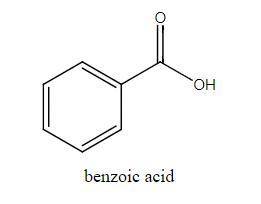

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 23.06.2019 09:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2

Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1
You know the right answer?
The molar mass of benzoic acid (C6H5COOH) determined by measuring the freezing‐point depression in b...
Questions

Mathematics, 24.01.2020 18:31

Computers and Technology, 24.01.2020 18:31



World Languages, 24.01.2020 18:31

Social Studies, 24.01.2020 18:31



Mathematics, 24.01.2020 18:31



History, 24.01.2020 18:31










