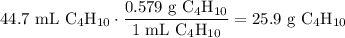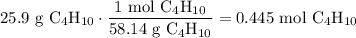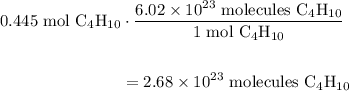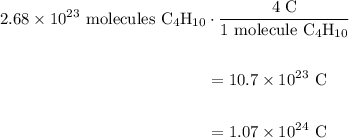Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Who is better, messi or cristiano, i need this for a chemistry class. asap
Answers: 1

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1


Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
A beaker contains 44.7 mL of butane ( C4H10, density is 0.579 g/mL).
Determine how many C atoms th...
Questions

Spanish, 26.08.2020 17:01




Chemistry, 26.08.2020 17:01


Mathematics, 26.08.2020 17:01

English, 26.08.2020 17:01






Chemistry, 26.08.2020 17:01






Mathematics, 26.08.2020 17:01