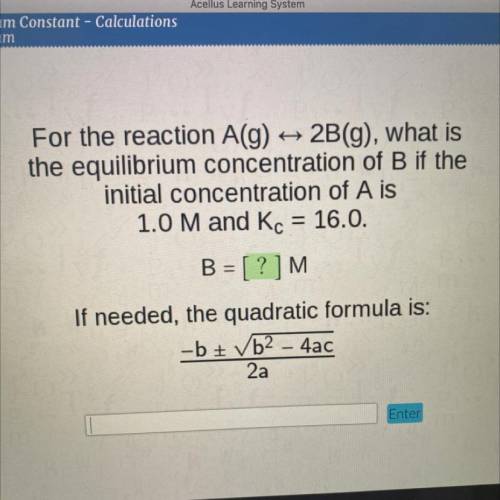
For the reaction A(g)
2B(g), what is
the equilibrium concentration of B if the
initial...

For the reaction A(g)
2B(g), what is
the equilibrium concentration of B if the
initial concentration of A is
1.0 M and Kc = 16.0.
B = [?]M
If needed, the quadratic formula is:
-b + b2 - 4ac
2a
PLEASE HELP IM SO CONFUSED AND NO LESSON VIDEOS ARE HELPFULL
ILL RATE YOU BRAINLIEST

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
Questions

Mathematics, 11.03.2020 16:50

Social Studies, 11.03.2020 16:50

History, 11.03.2020 16:50



Computers and Technology, 11.03.2020 16:50

Mathematics, 11.03.2020 16:51



History, 11.03.2020 16:51

History, 11.03.2020 16:51


Biology, 11.03.2020 16:51







Mathematics, 11.03.2020 16:52



