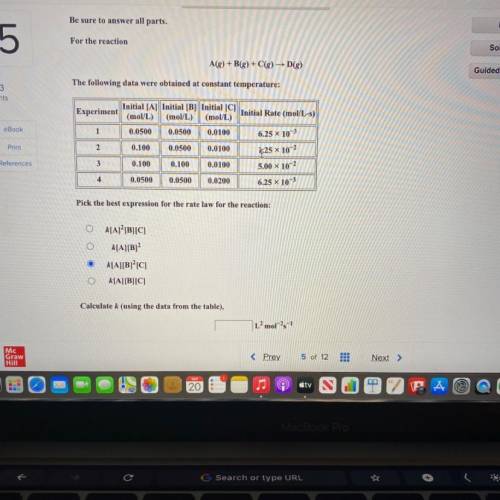
Be sure to answer all parts.
For the reaction
A(g) +B(g) + C(g) D()
The following data...

Be sure to answer all parts.
For the reaction
A(g) +B(g) + C(g) D()
The following data were obtained at constant temperature:
Initial [A] Initial [B] Initial [C]
Experiment
Initial Rate (mol/L-s)
(mol/L) (mol/L) (mol/L)
1 0.0500 0.0500 0.0100
6.25 x 103
2 0.100 0.0500 0.0100
3 0.100 0.100 0.0100 5.00 x 10-2
4 0.0500 0.0500 0.0200
6.25 x 10-3
1,25 x 10-2
Pick the best expression for the rate law for the reaction:
k[A] [B][C]
k[A][B]
k[A][B]°C]
k[A][B][C]
Calculate k (using the data from the table),
L’mol-1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

You know the right answer?
Questions


Mathematics, 28.02.2021 22:20



English, 28.02.2021 22:20

Mathematics, 28.02.2021 22:20

Chemistry, 28.02.2021 22:20


Mathematics, 28.02.2021 22:20


Mathematics, 28.02.2021 22:20

Chemistry, 28.02.2021 22:20

Mathematics, 28.02.2021 22:20


Spanish, 28.02.2021 22:20

Chemistry, 28.02.2021 22:20




Mathematics, 28.02.2021 22:20



