Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

You know the right answer?
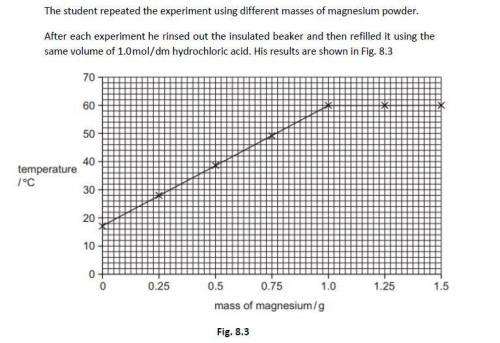
Explain, in terms of energy, why the temperature of the reaction mixture increases when magnesium po...
Questions

Mathematics, 08.04.2021 23:40

Social Studies, 08.04.2021 23:40



Mathematics, 08.04.2021 23:40

Chemistry, 08.04.2021 23:40



Mathematics, 08.04.2021 23:40

Mathematics, 08.04.2021 23:40

Social Studies, 08.04.2021 23:40

Chemistry, 08.04.2021 23:40



Mathematics, 08.04.2021 23:40


Chemistry, 08.04.2021 23:40

Biology, 08.04.2021 23:40

English, 08.04.2021 23:40

Mathematics, 08.04.2021 23:40