
Chemistry, 21.09.2021 03:30 azireyathurmond1
Select the correct answer.
Identify Bohr's model of the atom. His model describes the reactivity of an element based on its number of valence electrons.
A- "A"
B-"B"
C- "C"
D- "D"
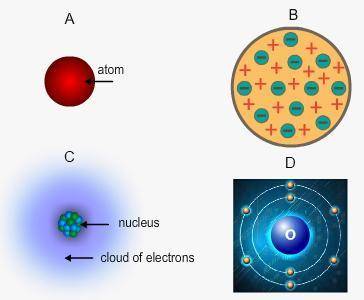

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
Select the correct answer.
Identify Bohr's model of the atom. His model describes the reactivity o...
Questions

Mathematics, 22.02.2020 23:05

Chemistry, 22.02.2020 23:05



Mathematics, 22.02.2020 23:06

Mathematics, 22.02.2020 23:06

Biology, 22.02.2020 23:06


English, 22.02.2020 23:07

Mathematics, 22.02.2020 23:08


Computers and Technology, 22.02.2020 23:08



Mathematics, 22.02.2020 23:09

Computers and Technology, 22.02.2020 23:10



History, 22.02.2020 23:11



