
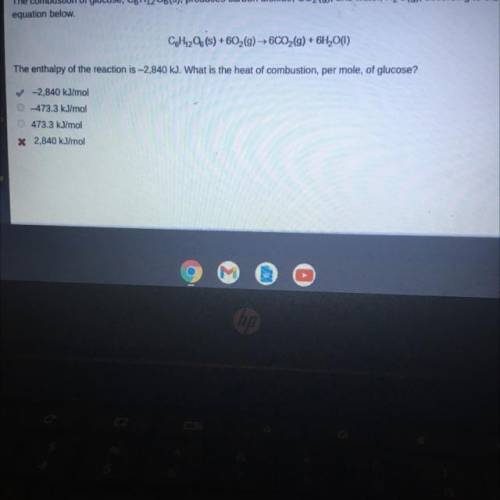
The combustion of glucose, C6H1206(s), produces carbon dioxide, CO2(g), and water, H2O(g), according to the
equation below.
CoH1206(s) +602(g) → 6CO2(g) + 6H2O(1)
The enthalpy of the reaction is -2,840 kJ. What is the heat of combustion, per mole, of glucose?
✓-2,840 kJ/mol
473.3 kJ/mol
473.3 kJ/mol
* 2,840 kJ/mol
See

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1
You know the right answer?
The combustion of glucose, C6H1206(s), produces carbon dioxide, CO2(g), and water, H2O(g), according...
Questions

English, 17.05.2021 20:00

History, 17.05.2021 20:00

Physics, 17.05.2021 20:00

Mathematics, 17.05.2021 20:00

Mathematics, 17.05.2021 20:00

History, 17.05.2021 20:00

Mathematics, 17.05.2021 20:00

Mathematics, 17.05.2021 20:00

Engineering, 17.05.2021 20:00

Mathematics, 17.05.2021 20:00


History, 17.05.2021 20:00



Geography, 17.05.2021 20:00

Mathematics, 17.05.2021 20:00



Mathematics, 17.05.2021 20:00

Mathematics, 17.05.2021 20:00



