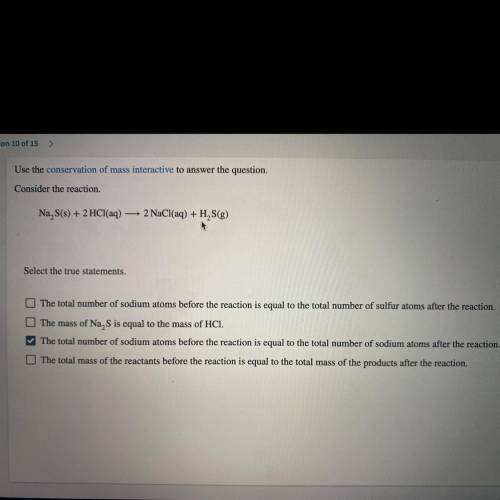
Use the conservation of mass interactive to answer the question.
Consider the reaction.
Na S...

Chemistry, 19.09.2021 06:20 Emptypockets451
Use the conservation of mass interactive to answer the question.
Consider the reaction.
Na S(s) + 2 HCl(aq) — 2 NaCl(aq) + H2S(g)
*Select the true statements.*
A. The total number of sodium atoms before the reaction is equal to the total number of sulfur atoms after the reaction.
B. The mass of Na, S is equal to the mass of HCI.
C. The total number of sodium atoms before the reaction is equal to the
total number of sodium atoms after the reaction.
D. The total mass of the reactants before the va reaction is equal to the total mass of the products after the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
Questions




Computers and Technology, 22.04.2020 02:51







Mathematics, 22.04.2020 02:51


Mathematics, 22.04.2020 02:51

Mathematics, 22.04.2020 02:51

Mathematics, 22.04.2020 02:51





Biology, 22.04.2020 02:52



