
Chemistry, 18.09.2021 01:00 annemcnair217
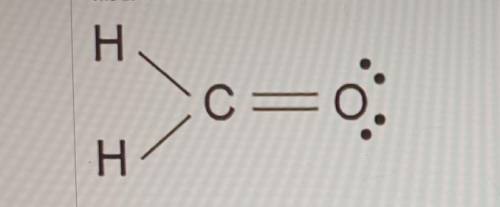
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 points)
O Oxygen is the least electronegative of the three atoms.
O Carbon has a total of four bonded pairs of electrons around it.
O Oxygen has four pairs of non-bonding innermost shell electrons.
O Carbon has an incomplete octet as it transfers an electron to each hydrogen.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 p...
Questions


History, 02.11.2019 23:31

Mathematics, 02.11.2019 23:31



Mathematics, 03.11.2019 00:31




Social Studies, 03.11.2019 00:31





Mathematics, 03.11.2019 00:31


Mathematics, 03.11.2019 00:31

Mathematics, 03.11.2019 00:31

Mathematics, 03.11.2019 00:31

Mathematics, 03.11.2019 00:31



