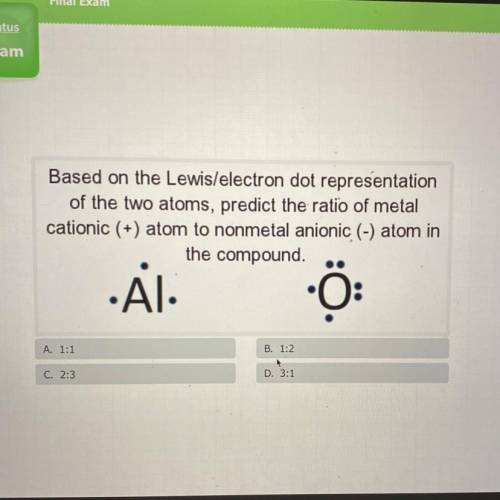
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
Questions



Arts, 21.04.2021 06:00

Mathematics, 21.04.2021 06:00

Social Studies, 21.04.2021 06:00



Biology, 21.04.2021 06:00

Mathematics, 21.04.2021 06:00

English, 21.04.2021 06:00

Mathematics, 21.04.2021 06:00

History, 21.04.2021 06:00

Mathematics, 21.04.2021 06:00






Social Studies, 21.04.2021 06:00