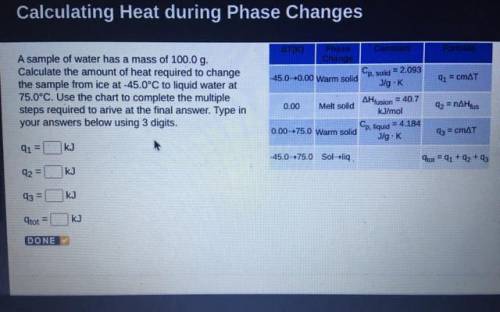
A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the...

A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the sample from ice at -45.0°C to liquid water at
75.0°C. Use the chart to complete the multiple
steps required to arive at the final answer. Type in
your answers below using 3 digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
Questions



Mathematics, 13.01.2021 02:20

Biology, 13.01.2021 02:20


Mathematics, 13.01.2021 02:20

Mathematics, 13.01.2021 02:20

Computers and Technology, 13.01.2021 02:20


Chemistry, 13.01.2021 02:20


Arts, 13.01.2021 02:20


Mathematics, 13.01.2021 02:20

Mathematics, 13.01.2021 02:20

Social Studies, 13.01.2021 02:20

History, 13.01.2021 02:20

Mathematics, 13.01.2021 02:20

Mathematics, 13.01.2021 02:20

Mathematics, 13.01.2021 02:20



