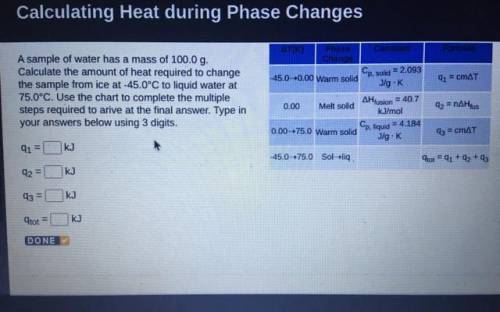
A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the...

Chemistry, 08.09.2021 02:40 accounting73
A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the sample from ice at -45.0°C to liquid water at
75.0°C. Use the chart to complete the multiple
steps required to arive at the final answer. Type in
your answers below using 3 digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
Questions



Business, 23.08.2020 01:01


Mathematics, 23.08.2020 01:01


Business, 23.08.2020 01:01

Spanish, 23.08.2020 01:01

Mathematics, 23.08.2020 01:01

Mathematics, 23.08.2020 01:01

Mathematics, 23.08.2020 01:01


Mathematics, 23.08.2020 01:01



Chemistry, 23.08.2020 01:01


Social Studies, 23.08.2020 01:01




