Use the References to access important values if needed for this question.
H
A-Z
The p...

Chemistry, 06.09.2021 05:50 destiny465
Use the References to access important values if needed for this question.
H
A-Z
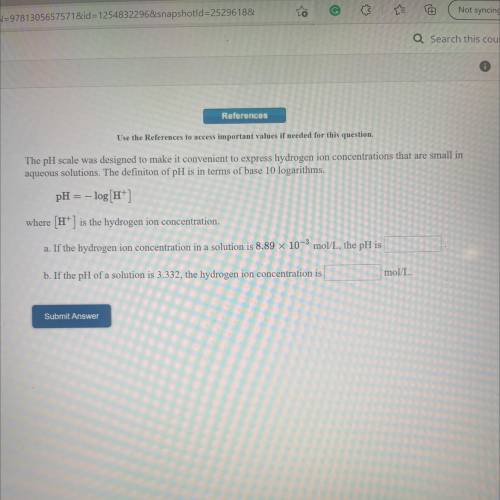
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in
aqueous solutions. The definiton of pH is in terms of base 10 logarithms.
pH = -log[H+]
where (H+) is the hydrogen ion concentration.
a. If the hydrogen ion concentration in a solution is 8.89 x 10-3 mol/L, the pH is
b. If the pH of a solution is 3.332, the hydrogen ion concentration is
mol/L.
Submit Answer

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
Questions

Computers and Technology, 30.07.2019 21:30

Mathematics, 30.07.2019 21:30

History, 30.07.2019 21:30


Mathematics, 30.07.2019 21:30


Mathematics, 30.07.2019 21:30

Health, 30.07.2019 21:30


Mathematics, 30.07.2019 21:30





Mathematics, 30.07.2019 21:30

Mathematics, 30.07.2019 21:30



Computers and Technology, 30.07.2019 21:30



