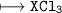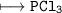---
An atom of element X contains 15 electrons and 16 neutrons.
(a). (I) State the mass...

Chemistry, 05.09.2021 07:50 jamisoncameron000
---
An atom of element X contains 15 electrons and 16 neutrons.
(a). (I) State the mass number of X.
(ii) Write the electronic
configuration of X.
(b) (I) Write the formula of a chloride of X. pliz , it's urgent.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
Questions



Mathematics, 22.01.2021 22:10





English, 22.01.2021 22:10

Mathematics, 22.01.2021 22:10

Mathematics, 22.01.2021 22:10






Mathematics, 22.01.2021 22:10


Mathematics, 22.01.2021 22:10


World Languages, 22.01.2021 22:10

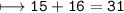
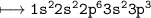 .
.