
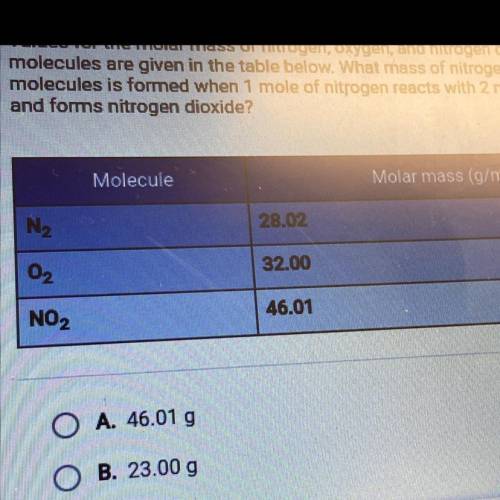
Values for the molar mass of nitrogen, oxygen, and nitrogen dioxide
molecules are given in the table below. What mass of nitrogen dioxide
molecules is formed when 1 mole of nitrogen reacts with 2 moles of oxygen
and forms nitrogen dioxide?
A. 46.01 g
B. 23.00 g
C. 2.00 g
D. 92.02 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Values for the molar mass of nitrogen, oxygen, and nitrogen dioxide
molecules are given in the tab...
Questions








Mathematics, 09.12.2021 02:30

Mathematics, 09.12.2021 02:30



Computers and Technology, 09.12.2021 02:30

Mathematics, 09.12.2021 02:30



Mathematics, 09.12.2021 02:30

History, 09.12.2021 02:30



Advanced Placement (AP), 09.12.2021 02:30



