A piece of zinc metal whose mass is 1.00
gram was dropped into some dilute sulfu-
ric acid m...

Chemistry, 31.08.2021 21:40 willjean6978
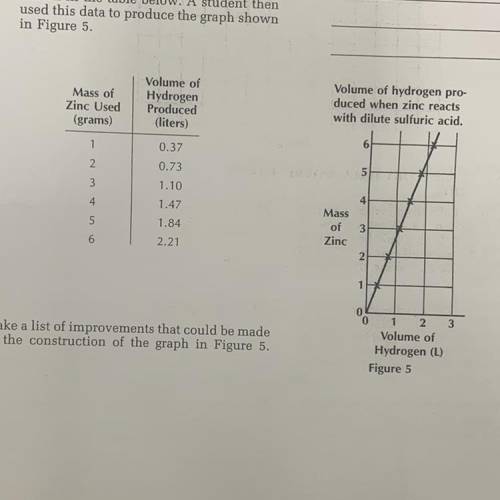
A piece of zinc metal whose mass is 1.00
gram was dropped into some dilute sulfu-
ric acid made by adding 10 grams of con-
centrated sulfuric acid to enough water to
make 100 milliliters of the acid solution.
After a while, all the zinc was "eaten
away” by the acid. An amount of hydrogen
equal to 0.37 liter was produced by the
reaction. The experiment was repeated
five more times, each time with the same
volume of the same sulfuric acid solution
but with pieces of zinc having different
masses. The results of the experiment are
shown in the table below. A student then
used this data to produce the graph shown
in Figure 5.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1


You know the right answer?
Questions

Geography, 22.05.2020 00:13






Social Studies, 22.05.2020 00:13




History, 22.05.2020 00:13

Mathematics, 22.05.2020 00:13

Biology, 22.05.2020 00:13




History, 22.05.2020 00:13


Mathematics, 22.05.2020 00:13




