
Chemistry, 30.08.2021 04:20 barstr9146
You have prepared a .2 M histidine solution. Calculate the molar concentration of isoelectric histidine at a) pH 5.5 and b) pH 7.0. A solution is provided, but can you do a step-by-step as I do not quite understand the math that went into this.
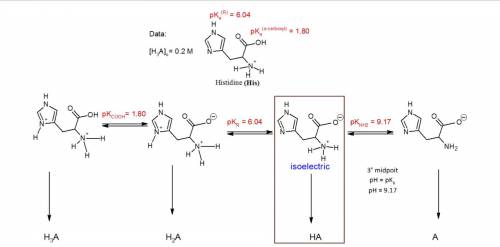
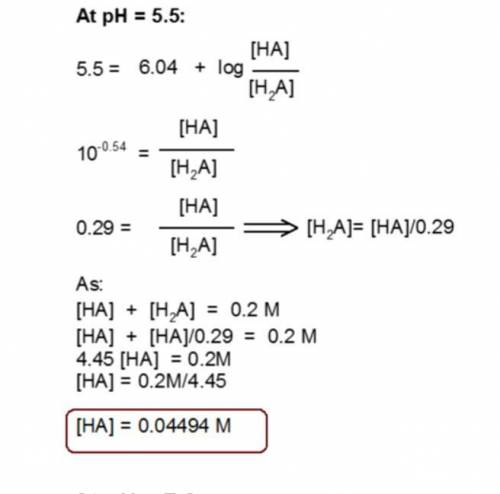

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
You have prepared a .2 M histidine solution. Calculate the molar concentration of isoelectric histid...
Questions

Mathematics, 29.07.2020 01:01






Mathematics, 29.07.2020 01:01

Mathematics, 29.07.2020 01:01










English, 29.07.2020 01:01

Mathematics, 29.07.2020 01:01

Arts, 29.07.2020 01:01



