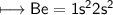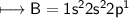Chemistry, 28.08.2021 16:30 coolfab9338
3. What is the best explanation for the decrease in first ionization energy moving from Be to B?
(A) Moving from Be to B, more electrons are added to the
atoms, thus increasing the electron-electron repulsions.
(B) Moving from Be to B, more protons in the nucleus
attract the valence electrons, making it harder to remove
the electrons.
(C) The electrons in Be are being removed from a full sub- shell, which is more stable than the half-filled sub-shell in B.
(D) The electrons in Be are located in the 2s subshell. These electrons penetrate closer to the nucleus than the 2p electrons in B.


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1


Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
3. What is the best explanation for the decrease in first ionization energy moving from Be to B?
(...
Questions



Mathematics, 11.05.2021 18:50

English, 11.05.2021 18:50



Mathematics, 11.05.2021 18:50

Physics, 11.05.2021 18:50


Health, 11.05.2021 19:00


Mathematics, 11.05.2021 19:00


Physics, 11.05.2021 19:00



Mathematics, 11.05.2021 19:00



Law, 11.05.2021 19:00