
Chemistry, 27.08.2021 08:10 emblemhacks
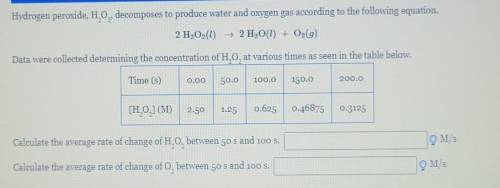
Hydrogen peroxide, H, O,,decomposes to produce water and oxygen gas according to the following equation. 2 H2O2(1) + 2 H2O(l) + O2(g) Data were collected determining the concentration of H20, at various times as seen in the table below. Time (s) 0.00 50.0 100.0 150.0 200.0 [H, O,](M) 2.50 1.25 0.625 0.46875 0.3125 Calculate the average rate of change of H,0, between 50 s and 100 s. OM/S Calculate the average rate of change of O, between 50 s and 100 s. OM/s

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Hydrogen peroxide, H, O,,decomposes to produce water and oxygen gas according to the following equat...
Questions

History, 14.03.2022 17:50


Mathematics, 14.03.2022 17:50



Biology, 14.03.2022 18:00


Mathematics, 14.03.2022 18:00





Mathematics, 14.03.2022 18:00






History, 14.03.2022 18:00




