
Chemistry, 26.08.2021 23:00 FriendlyDude640
Calculate the percentage of limestone that dissolved from each solution. Start by subtracting the final mass from the initial mass. Divide that number by the initial mass. Then multiply the result by 100 to make it a percent. Use this formula:
% dissolved= initial mass- final mass/ initial mass x 100
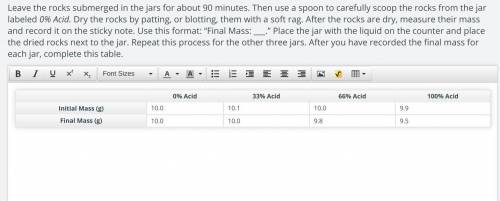
ORIGINAL: 0% Acid 33% Acid 66% Acid 100% Acid
Initial Mass (g) 10.0 10.1 10.0 9.9
Final Mass (g) 10.0 10.0 9.8 9.5
Record the percentage of limestone dissolved in each acid concentration.
Look at attched...

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
Calculate the percentage of limestone that dissolved from each solution. Start by subtracting the fi...
Questions


Mathematics, 17.10.2019 18:00

Social Studies, 17.10.2019 18:00


Mathematics, 17.10.2019 18:00

Chemistry, 17.10.2019 18:00



Chemistry, 17.10.2019 18:00


Mathematics, 17.10.2019 18:00

Arts, 17.10.2019 18:00


Mathematics, 17.10.2019 18:00



History, 17.10.2019 18:00



Mathematics, 17.10.2019 18:00



