What is the freezing point, in °C,
of a 1.56 m aqueous solution
of K3PO4?
Note: K for...

Chemistry, 25.08.2021 20:10 kyahshayovvu24
What is the freezing point, in °C,
of a 1.56 m aqueous solution
of K3PO4?
Note: K for water is 1.86 °C/m
Enter rounded answer to three decimal points.
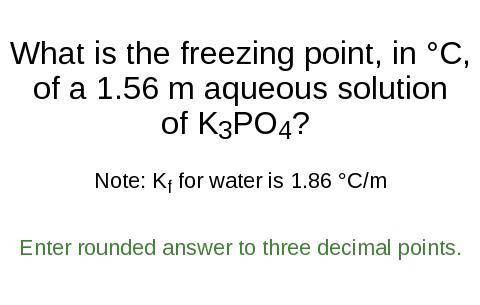

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2

You know the right answer?
Questions


Social Studies, 22.11.2019 04:31

Chemistry, 22.11.2019 04:31




History, 22.11.2019 04:31




History, 22.11.2019 04:31

Mathematics, 22.11.2019 04:31

Geography, 22.11.2019 04:31


Mathematics, 22.11.2019 04:31

Geography, 22.11.2019 04:31




History, 22.11.2019 04:31



