Chemistry, 21.08.2021 02:00 garciaarmando13
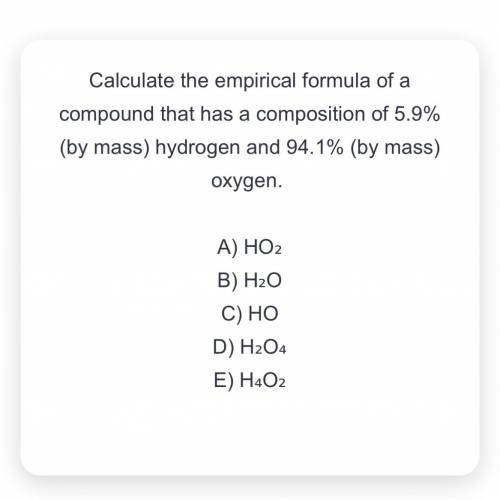
Calculate the empirical formula of a compound that has a composition of 5.9% (by mass) hydrogen and 94.1% (by mass) oxygen.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

You know the right answer?
Calculate the empirical formula of a compound that has a composition of 5.9% (by mass) hydrogen and...
Questions


Mathematics, 29.09.2021 22:20

Mathematics, 29.09.2021 22:20




Chemistry, 29.09.2021 22:20

Mathematics, 29.09.2021 22:20

History, 29.09.2021 22:20


Mathematics, 29.09.2021 22:20

Mathematics, 29.09.2021 22:20

Mathematics, 29.09.2021 22:20


English, 29.09.2021 22:20


Mathematics, 29.09.2021 22:20

Chemistry, 29.09.2021 22:20


Chemistry, 29.09.2021 22:20