
Please help!
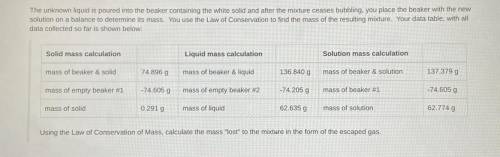
The unknown liquid is poured into the beaker containing the white solid and after the mixture ceases bubbling, you place the beaker with the new
solution on a balance to determine its mass. You use the Law of Conservation to find the mass of the resulting mixture. Your data table, with all
data collected so far is shown below:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 09:20
Four statements about the development of the atomic model are shown below. a: electrons have wavelike properties. b: atoms have small, negatively charged particles. c. the center of an atom is a small, dense nucleus. d: atoms are hard, indivisible spheres. which order of statements represents the historical development of the atomic model? c-d-a-b c-d-b-a d— в-а — с d-b-c-a
Answers: 1

Chemistry, 23.06.2019 18:20
Consider the following system at equilibrium. caco3(s) = ca2+(aq) + co32-(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? occia co2 cuso4 na2co3
Answers: 3

Chemistry, 23.06.2019 19:30
Is the following chemical equation balanced? agno3 + nacl 4agcl + nano3 yes no
Answers: 1
You know the right answer?
Please help!
The unknown liquid is poured into the beaker containing the white solid and after the...
Questions

Social Studies, 20.08.2019 23:30

Biology, 20.08.2019 23:30

Chemistry, 20.08.2019 23:30



Mathematics, 20.08.2019 23:30


History, 20.08.2019 23:30





Chemistry, 20.08.2019 23:30

Chemistry, 20.08.2019 23:30



Mathematics, 20.08.2019 23:30

Physics, 20.08.2019 23:30


History, 20.08.2019 23:30



